Main Article Content
Abstract
In present scenario huge quantity of waste are produced every day. It contains plastics, waste papers, plastic containers, some of these are degradable, and some of these are non-degradable waste.Non-biodegradable things are present in waste treated with mechanical pulverizing mechanisms and with few of strong chemical. Chemicals such as concentrated Hydrochloric, Sulphuric acids, sulphamic acids and many other hazardous chemicals are used for destroying wastes in simple form or in disperse form.
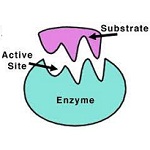
Almost 70% of the waste is being dumped in the soil layer. After certain interval of time compounds present in soils such as salts of the metals and temperature of soil help in decomposing the waste periodically. Due to this process large quantity of soil gets contaminated. In this project we have tried to replace hazardous chemicals with other non-hazardous chemicals and some natural enzymes, which may give same reactions as with toxic chemicals. Objectives may also helps in use of eco friendly chemicals for reducing soil pollution and water pollutions. Hence it will minimize the other impacts on environment such as air pollution, noise pollutions, water pollutions and soil pollutions. All process will carry out under Green Chemistry cycles.
Keywords
Article Details
Authors retain the copyright without restrictions for their published content in this journal. GCTL is a Sherpa Romeo Journal.
Publishing License
This is an open-access article distributed under the terms of 
References
- Cai Y, M Sun, and H Corke. Antioxidant activity of betalains from plants of the Amaranthaceae. J Agric Food Chem 2003; 51(8):2288-94. DOI: https://doi.org/10.1021/jf030045u
- Törrönen, R. Sources and health effects of dietary ellagitannins. In Chemistry and Biology ofEllagitannins, An Underestimated Class of Bioactive Plant Polyphenols; Quideau, S., Ed.; WorldScientific: Singapore, 2009; pp. 298–315. DOI: https://doi.org/10.1142/9789812797414_0008
- Guyot , S.; Vercauteren, J.; Cheynier, V. Structural determination of colourless and yellowdimmers resulting from (+)-catechin coupling catalysed by grape polyphenoloxidase.Phytochemistry 1996, 42, 1279–1288. DOI: https://doi.org/10.1016/0031-9422(96)00127-6
- Lopez-Serrano, M.; Ros Barcelo, A. Reversed-phase and size-exclusion chromatography as useful tools in the resolution of peroxidase-mediated (+)-catechin oxidation products. J.Chromatogr. A 2001, 919, 267–273. DOI: https://doi.org/10.1016/S0021-9673(01)00817-2
- Tanaka, T.; Betsumiya, Y.; Mine, C.; Kouno, I. Theanaphthoquinone, a novel pigmentoxidatively derived from theaflavin during tea-fermentation. Chem. Commune. 2000, 1365–1366. DOI: https://doi.org/10.1039/b003510f
- Jhoo, J.-W.; Lo, C.-Y.; Li, S.; Sang, S.; Ang, C.Y.W.; Heinze, T.M.; Ho, C.-T. Stability of black.
- Tanaka, T.; Inoue, K.; Betsumiya, Y.; Mine, C.; Kouno, I. Two types of oxidative dimerizationof the black tea polyphenol theaflavin. J. Agric. Food Chem. 2001, 49, 5785–5789. DOI: https://doi.org/10.1021/jf010842x
- Lewis, J.R.; Davis, A.L.; Cai, Y.; Davies, A.P.; Wilkins, J.P.G.; Pennington, M. Theaflavate B,isotheaflavin-3'-O-gallate and neotheaflavin-3-O-gallate: Three polyphenolic pigments from black tea. Phytochemistry 1998, 49, 2511–2519. DOI: https://doi.org/10.1016/S0031-9422(98)00153-8
- Hashimoto, F.; Nonaka, G.; Nishioka, I. Tannins and related compounds. LXIX. Isolation and structure elucidation of B,B'-linked bisflavanoids, theasinensins D-G and oolongtheanin from oolong tea (2). Chem. Pharm. Bull. 1988, 3. DOI: https://doi.org/10.1248/cpb.36.1676
References
Cai Y, M Sun, and H Corke. Antioxidant activity of betalains from plants of the Amaranthaceae. J Agric Food Chem 2003; 51(8):2288-94. DOI: https://doi.org/10.1021/jf030045u
Törrönen, R. Sources and health effects of dietary ellagitannins. In Chemistry and Biology ofEllagitannins, An Underestimated Class of Bioactive Plant Polyphenols; Quideau, S., Ed.; WorldScientific: Singapore, 2009; pp. 298–315. DOI: https://doi.org/10.1142/9789812797414_0008
Guyot , S.; Vercauteren, J.; Cheynier, V. Structural determination of colourless and yellowdimmers resulting from (+)-catechin coupling catalysed by grape polyphenoloxidase.Phytochemistry 1996, 42, 1279–1288. DOI: https://doi.org/10.1016/0031-9422(96)00127-6
Lopez-Serrano, M.; Ros Barcelo, A. Reversed-phase and size-exclusion chromatography as useful tools in the resolution of peroxidase-mediated (+)-catechin oxidation products. J.Chromatogr. A 2001, 919, 267–273. DOI: https://doi.org/10.1016/S0021-9673(01)00817-2
Tanaka, T.; Betsumiya, Y.; Mine, C.; Kouno, I. Theanaphthoquinone, a novel pigmentoxidatively derived from theaflavin during tea-fermentation. Chem. Commune. 2000, 1365–1366. DOI: https://doi.org/10.1039/b003510f
Jhoo, J.-W.; Lo, C.-Y.; Li, S.; Sang, S.; Ang, C.Y.W.; Heinze, T.M.; Ho, C.-T. Stability of black.
Tanaka, T.; Inoue, K.; Betsumiya, Y.; Mine, C.; Kouno, I. Two types of oxidative dimerizationof the black tea polyphenol theaflavin. J. Agric. Food Chem. 2001, 49, 5785–5789. DOI: https://doi.org/10.1021/jf010842x
Lewis, J.R.; Davis, A.L.; Cai, Y.; Davies, A.P.; Wilkins, J.P.G.; Pennington, M. Theaflavate B,isotheaflavin-3'-O-gallate and neotheaflavin-3-O-gallate: Three polyphenolic pigments from black tea. Phytochemistry 1998, 49, 2511–2519. DOI: https://doi.org/10.1016/S0031-9422(98)00153-8
Hashimoto, F.; Nonaka, G.; Nishioka, I. Tannins and related compounds. LXIX. Isolation and structure elucidation of B,B'-linked bisflavanoids, theasinensins D-G and oolongtheanin from oolong tea (2). Chem. Pharm. Bull. 1988, 3. DOI: https://doi.org/10.1248/cpb.36.1676